Answer:
Empirical formula of a compound means that it provides simplest ratio of whole number.
Step-by-step explanation:
QUESTION-1
Given,
Mass of boron and chlorine is 9.224% and 90.74%
Then, Boron- 9.224×
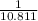 =0.85 mol (Mass value of boron=10.811)
=0.85 mol (Mass value of boron=10.811)
Chlorine- 90.74×
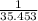 =2.559 mol.(Mass value of chlorine=35.453)
=2.559 mol.(Mass value of chlorine=35.453)
By dividing the mole value with smallest number, then we will get-
Boron-
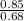 =1
=1
Chlorine-
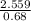 =3
=3
The empirical formula of the compound is -BCl₃
QUESTION-2
BCl₃⇄B+ 3.Cl (Putting the actual mass number of boron and chlorine we will get-)
1×(10.811)+ 3×(35.453)=117.17g/mol
Molecular formula=
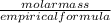
=
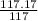 ≈1
≈1
The molecular formula is same with the empirical formula.