Answer:
0.7447%
Step-by-step explanation:
From the diagram below:
We consider the
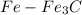 phase transformation
phase transformation
From the lever rule, mass fraction of eutectoid ferrite in a hypo eutectoid iron-carbon alloy
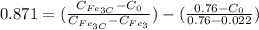
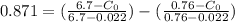
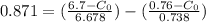

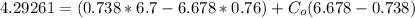
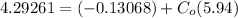
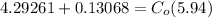
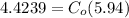
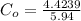
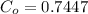 % of C
% of C
Hence, the weight% of Carbon in the alloy is
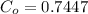 % of C.
% of C.