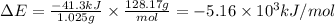Answer:
-5.16 × 10³ kJ/mol
Step-by-step explanation:
According to the law of conservation of energy, the sum of the heat released by the combustion of naphthalene (Qcomb) and the heat absorbed by the bomb calorimeter (Qcal) is zero.
Qcomb + Qcal = 0
Qcomb = -Qcal [1]
We can calculate the heat absorbed by the bomb calorimeter using the following expression:
Qcal = Ccal × ΔT
where,
- Ccal: heat capacity of the calorimeter
- ΔT: change in the temperature
Qcal = Ccal × ΔT = 5.11 kJ/°C × (32.33°C-24.25°C) = 41.3 kJ
From [1],
Qcomb = -41.3 kJ
41.3 kJ are released upon the combustion of 1.025 g of naphthalene (MW 128.17). The change in the internal energy (ΔE) is: