Answer:
The thermal energy increases
Step-by-step explanation:
The thermal energy is the total energy contained in a substance due to the thermal motion of its molecules.
In particular, the thermal energy of a substance is directly proportional to:
- The mass of the substance (the more the mass, the more the thermal energy)
- The average kinetic energy of the molecules of the substance (which is directly proportional to the temperature of the substance)
Therefore, we can write:
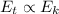
where
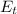 is the thermal energy of the substance
is the thermal energy of the substance
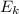 is the average kinetic energy of the molecules
is the average kinetic energy of the molecules
Therefore, we see that if the average kinetic energy of the molecules goes up, then the thermal energy increases as well.