Answer:
(4)
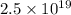 more protons than electrons
more protons than electrons
Step-by-step explanation:
Total positive change on the object is given as
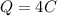
now by the law of quantization of charge we know that the charge on any body is always in form of integral multiple of charge of an electron.
So we will have
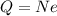
here we know that

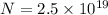
Now we know that the object is positively charged so here electrons must be less in numbers than protons
so correct answer will be
(4)
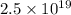 more protons than electrons
more protons than electrons