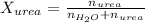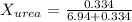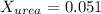Answer : The molecular wight of urea is, 59.9 g/mol
Mole fraction of water and urea is, 0.95 and 0.051 respectively.
Explanation :
According to the relative lowering of vapor pressure, the vapor pressure of a component at a given temperature is equal to the mole fraction of that component of the solution multiplied by the vapor pressure of that component in the pure state.
Formula used :
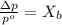
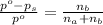
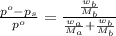
where,
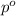 = vapor pressure of the pure solvent water = 23.76 torr
= vapor pressure of the pure solvent water = 23.76 torr
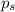 = vapor pressure of the solution = 22.67 torr
= vapor pressure of the solution = 22.67 torr
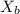 = mole fraction of solute (urea)
= mole fraction of solute (urea)
n is the number of moles
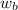 = mass of urea = 20.0 g
= mass of urea = 20.0 g
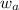 = mass of water = 125 g
= mass of water = 125 g
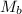 = molar mass of urea = ?
= molar mass of urea = ?
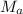 = molar mass of water = 18 g/mol
= molar mass of water = 18 g/mol
Now put all the given values in the above expression, we get:
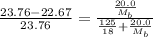
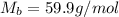
Now we have to calculate the moles of
 and urea.
and urea.
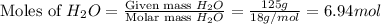
and,
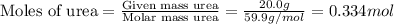
Now we have to calculate the mole fraction of
 and urea.
and urea.
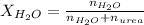
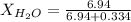
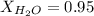
and,