Answer:
145 ° C
Step-by-step explanation:
Using the expression
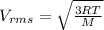
where T = 17 ° C = (273 + 17) K
= 290 K
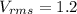
So; At what temperature will its root-mean-square speed (thermal speed) be 1.2 times its value at 17.0 deg C?
What this implies is that:
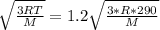 where R & M are constant
where R & M are constant
T = 144.6 ° C
T = 145 ° C