40 ppm of NaOH
Step-by-step explanation:
ppm is nothing but the parts per million and it is represented as 1 mg solute per kg of solution.
Here, number of moles of solute (NaOH) can be found as 0.001 mol / L, so we can find the amount of solute as,
Moles =
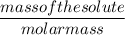
0.001 mol / L =
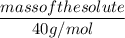
0.001 × 40 = 0.04 g of solute
1 g = 1000 mg
0.04 g = 40 mg
In 1 kg of solution 40 mg of solvent is present.
So the concentration of NaOH is 40 ppm.