Answer: The partial pressure of the dry oxygen is 742 torr
Step-by-step explanation:
Dalton's Law of Partial Pressure states that the total pressure exerted by a mixture of gases is the sum of partial pressure of each individual gas present. Thus
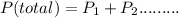
Given; Total pressure = 762 torr
partial pressure of water = 19.8 torr
partial pressure of dry oxygen = ? torr
Total pressure = partial pressure of water + partial pressure of dry oxygen
762 torr = 19.8 torr = partial pressure of dry oxygen
partial pressure of dry oxygen = 742 torr
The partial pressure of the dry oxygen is 742 torr