The given question is incomplete. The complete question is :
What is the molarity of a solution containing 3.0 moles of silver chloride dissolved in enough water to make 2.0 liter solution.
Answer: Molarity of a solution containing 3.0 moles of silver chloride dissolved in enough water to make 2.0 liter solutuion is 1.5 M
Step-by-step explanation:
Molarity is defined as the number of moles of solute dissolved per liter of the solution.
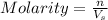
where,
n= moles of solute (silver chloride) = 3.0
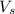 = volume of solution in L= 2.0 L
= volume of solution in L= 2.0 L
Putting in the values we get:
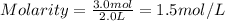
Thus molarity of a solution containing 3.0 moles of silver chloride dissolved in enough water to make 2.0 liter solutuion is 1.5 M