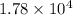Answer : The value of 'K' is,
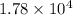
Explanation :
The copper(II) ion reacts with phosphate ion to form an insoluble ionic compound.
(1) The chemical reaction will be:
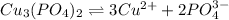
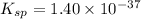
The copper(II) ion also forms a complex ion with ammonia.
(2) The chemical reaction will be:
![Cu^(2+)+4NH_3\rightarrow [Cu(NH_3)_4]^(2+)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/mbz2acej8clzdopva4y1kxq6o3onxyzppx.png)
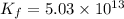
To calculate the value of 'K' we are multiplying reaction 2 by 3, we get:
(3)
![3Cu^(2+)+12NH_3\rightarrow 3[Cu(NH_3)_4]^(2+)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/y73d4f3vw7nrmy0dvcsgl72q74vpp8hy6h.png)
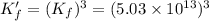
Now we are adding reaction 1 and 3, we get:
![Cu_3(PO_4)_2+12NH_3\rightarrow [Cu(NH_3)_4]^(2+)+2PO_4^(3-)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/4yb03g2nxhs80igcv375wxnezhurz2o9ce.png)
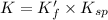
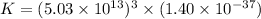
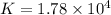
Therefore, the value of 'K' is,