Answer:
It will be 4 times larger.
Explanation:
The radius of a fluorine atom is about
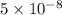 mm. and the radius of a strontium atom is about
mm. and the radius of a strontium atom is about
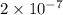 mm.
mm.
We have to calculate the number of times larger the radius of a strontium atom than the radius of a fluorine atom.
We will get the number by dividing the radius of a strontium atom by the radius of a fluorine atom.
Hence, it will be
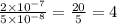 times larger. (Answer)
times larger. (Answer)