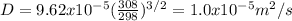Answer:
D=1.0x10^-5 m^2/s
Step-by-step explanation:
the data given by the exercise are as follows:
T=308 K
d=1 cm
PA=0.195 atm
pL=0.85 g/cm^3
The expression for binary gas-phase diffusion coefficient is equal to:
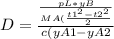
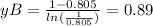
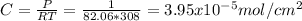
substituting the values in the diffusion equation:
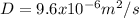
from Appendix J-1 from Welty:
298 K, D=9.62x10^-5 m^2/s
At 308 K, we have the following: