Answer:
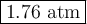
Step-by-step explanation:
The volume and amount of gas are constant, so we can use Gay-Lussac’s Law:
At constant volume, the pressure exerted by a gas is directly proportional to its temperature.
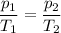
Data:
p₁ = 1.34 atm; T₁ = 237 K
p₂ = ?; T₂ = 312 K
Calculations:
