If you place 1.0 L of ethanol (C2H5OH) in a small laboratory that is 3.0 m long, 2.0 m wide, and 2.0 m high, will all the alcohol evaporate? If some liquid remains, how much will there be? The vapor pressure of ethyl alcohol at 25 °C is 59 mm Hg, and the density of the liquid at this temperature is 0.785g/cm^3 .
will all the alcohol evaporate? or none at all?
Answer:
Yes, all the ethanol present in the laboratory will evaporate since the mole of ethanol present in vapor is greater. The volume of ethanol left will therefore be zero.
Step-by-step explanation:
Given that:
The volume of alcohol which is placed in a small laboratory = 1.0 L
Vapor pressure of ethyl alcohol at 25 ° C = 59 mmHg
Converting 59 mmHg to atm ; since 1 atm = 760 mmHg;
Then, we have:
=
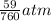
= 0.078 atm
Temperature = 25 ° C
= ( 25 + 273 K)
= 298 K.
Density of the ethanol = 0.785 g/cm³
The volume of laboratory = l × b × h
= 3.0 m × 2.0 m × 2.5 m
= 15 m³
Converting the volume of laboratory to liter;
since 1 m³ = 100 L; Then, we have:
15 × 1000 = 15,000 L
Using ideal gas equation to determine the moles of ethanol in vapor phase; we have:
PV = nRT
Making n the subject of the formula; we have:
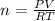
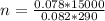
n = 47. 88 mol of ethanol
Moles of ethanol in 1.0 L bottle can be calculated as follows:
Since numbers of moles =

and mass = density × vollume
Then; we can say ;
number of moles =
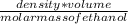
number of moles =
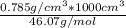
number of moles =
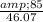
number of moles = 17.039 mol
Thus , all the ethanol present in the laboratory will evaporate since the mole of ethanol present in vapor is greater. The volume of ethanol left will therefore be zero.