Answer : The pOH of pure water is, 6.68
Explanation :
As we are given that:
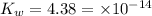
First we have to calculate the concentration of hydroxide ion.
As,
![K_w=[H^+]* [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/qakykjskdgnhxc6kp4ssjh380yeaa8gkx8.png)
As we know that in pure water the hydrogen ion and hydroxide ion concentration are equal. That means,
![[H^+]=[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/lp8fbaeok2xwhex5vwkyo0d1wjhj7t32mz.png)
So,
![K_w=[OH^-]* [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/7wzymekgpjjdmdhm39jnvvuyqd93v0dsk1.png)
![K_w=[OH^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/jwzwvff87lvw1ox6xb9v8jinb5tx1uxchk.png)
![4.38* 10^(-14)=[OH^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/qek31aj762r1nfy68t5t67qt0apt2z0hjj.png)
![[OH^-]=2.09* 10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/miv659zlgsoioqijjsidd2h4zdg71rkse5.png)
Now we have to calculate the pOH.
![pOH=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hdm1ob4dj6mx2sy3kobrrj91lzbh3927bk.png)
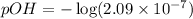
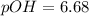
Therefore, the pOH of pure water is, 6.68