Answer: The equilibrium constant for the overall reaction is

Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.
a)
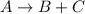
![K_1=([B]* [C])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/lb6m3ng23bkfqtm05k0pophgx4aqqougru.png)
b)
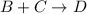
![K_2=([D])/([B]* [C])](https://img.qammunity.org/2021/formulas/chemistry/college/9yvl0leo5rqfqrr9d47ozow4lsut4dwmjk.png)
For overall reaction on adding a and b we get c
c)
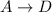
![K_3=([D])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/vxmweuky9ywyfxhjmz3cm4hq2fx3oo4l4r.png)
![K_3=K_1* K_2=([B]* [C])/([A])* ([D])/([B]* [C])](https://img.qammunity.org/2021/formulas/chemistry/college/ho9zj34km3rctmn2ph8zbcoq056p6a2rpp.png)
![K_3=K_1* K_2=([D])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/fft4y37nhhmxkcfc7qgsjrpy5kh2rps9ol.png)
The equilibrium constant for the overall reaction is
