Answer:
10.1 °C
Step-by-step explanation:
After the mixture, heat is gained by the soapstone cubes (they were at a lower temperature) while heat is lost by the whisky (it was at a higher temperature).
Let the temperature of the mixture be T.
Heat lost by whisky,
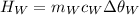
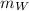 is the mass of the whisky (in kg),
is the mass of the whisky (in kg),
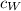 is the specific heat of whisky (J/kg·K) and
is the specific heat of whisky (J/kg·K) and
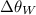 is the change in temperature of the whisky (in °C or K).
is the change in temperature of the whisky (in °C or K).
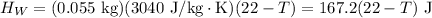
Heat gained by soapstone cubes,
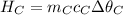
There are three cubes of soapstone, so their mass is multiplied by 3.
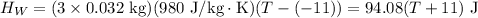
From the principle of mixtures,
Heat lost by whisky = Heat gained by soapstone cubes (assuming no heat loss to the surroundings)
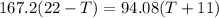
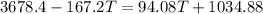
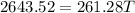
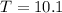
The final temperature of the whisky is 10.1 °C.