Answer:
115.95 grams of oxygen
Step-by-step explanation:
The reaction equations is expressed as:
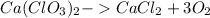
We know the molar mass of
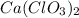 as
as
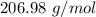 hence the number of moles in the 250g reaction is
hence the number of moles in the 250g reaction is
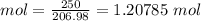
#Given the 1:3 ratio(to oxygen's moles), the number of moles of oxygen produced is:
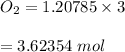
The molar mass of oxygen is 32g, therefore:
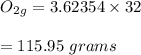
Hence, 115.95 grams of oxygen are produced.