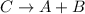Answer: synthesis and decomposition
Step-by-step explanation:
1. A double displacement reaction is one in which exchange of ions take place.
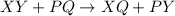
2. Combustion is a type of chemical reaction in which hydrocarbons burn in the presence of oxygen to form carbon dioxide and water along with heat.
3. A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.
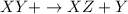
4. Synthesis is a type of chemical reaction when two reactants combine to give a single product.
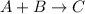
5. Decomposition reaction is a type of chemical reaction in which one reactant gives two or more than two products.