Answer:
- About 408g of KI can be dissolved in 300 g of water at 10ºC.
Step-by-step explanation:
Look at the graph attached. It contains the solubility curves for severals solutes.
I have marked the solubility of KI at 10ºC: look the intersection of the vertical and horizontal dotted red lines: the corresponding solubility is read on the vertical axis. It is slightlly above the midpoint between 130 y 140 g per 100 g of water. Thus, you can approximate to 136 g / 100 g of water.
You need the number of grams dissolved in 300 g of water, thus mutilply by 300:
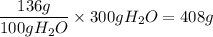
Thus, about 408g of KI can be dissolved in 300 g of water at 10ºC.