The answer for the following problem is explained below.
The specific heat of oil is 7.98 J/gram °C
Step-by-step explanation:
Given:
heat (q)=878 J
mass of oil (m) =5 grams
initial temperature (
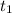 ) =34°C
) =34°C
final temperature (
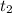 ) = 56°C
) = 56°C
To solve:
specific heat of the oil (c)
We know;
c =q÷(mΔT)
ΔT= (
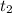 ) - (
) - (
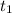 )
)
ΔT = 56°C - 34°C
ΔT = 22°C
c =
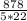
c =878÷ 110
c =7.98 J/gram °C
Therefore the specific heat of oil is 7.98 J/gram °C