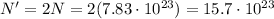Answer:

Step-by-step explanation:
In order to solve this problem, we have to find the number of moles of NaCl first.
The number of moles of NaCl is given by:
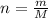
where:
m = 75.9 g is the mass of the sample of NaCl in this problem
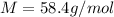 is the molar mass of NaCl, that is the amount of mass contained in 1 mole of the substance
is the molar mass of NaCl, that is the amount of mass contained in 1 mole of the substance
Therefore, we have
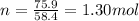
We also know that 1 mole of a substance contains always a number of molecules equal to the Avogadro number:
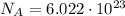
So, since here we have 1.30 moles, the number of molecules in this sample of NaCl is:
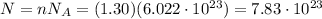
However, here we are asked how many atoms the sample contains. Since 1 molecule of NaCl contains 2 atoms (1 atom of Na and 1 atom of Cl), it means that the number of atoms in this sample is twice the number of molecules, so: