Answer:
Equilibrium constant Kc for the reaction will be 1.722
Step-by-step explanation:
O2(g)+NO(g)→CO(g)+ NO2(g)
0.88 3.9 --- ---
0.88x 3.9-x x x
GIVEN:
0.88X-X= 0.11
⇒ X=0.77
CO2(g)+NO(g) → CO(g) + NO2(g)
0.88 3.9 --- ---
0.88-x 3.9-x x x
= 3.13 0.77 0.77
=0.11
Kc =
![\frac{[CO] *[NO2]} {[CO2]*[NO]}](https://img.qammunity.org/2021/formulas/chemistry/high-school/w6b0me436d72mq4e0p5e0oi8t44metnvep.png)
=
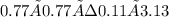
= 1.722