The total pressure exerted by the mixture of gases is 758 Torr.
Step-by-step explanation:
Dalton's law of partial pressure is used to determine the total pressure exerted by mixture of gases. Also if we know the total pressure and the partial pressure of N-1 gases for mixture of N gases, then we can determine the partial pressure for the Nth gas.
So as per that law, the total pressure of any mixture of gases is equal to the sum of the partial pressure of individual gases. As partial pressure is the measure or the ability of each gas to produce pressure in a given volume, so the sum of those partial pressure will give the total pressure present in the gas mixture.
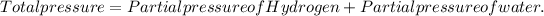
As the partial pressure of H₂ gas is given as 736 Torr and the partial pressure of H₂O is given as 22 Torr, the total pressure will be equal to the sum of those two pressures.
Total pressure = 736 + 22 = 758 Torr.
So, the total pressure exerted by the mixture of gases is 758 Torr.