7.33 grams of NaOH is the amount of conjugate base added.
Step-by-step explanation:
given that:
total volume = 150 ml
Vacid = 80 ml
Macid = 2.30 M
Vbase= 150-80= 70ml
Mbase=?
atomic mass of NaOH = 39.99 grams/mole
formula for titration
Macid X Vacid = Mbase X Vbase
putting the values in the equation:
Mbase =
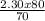
= 2.62M is the molarity of NaOH
Number of moles of
M =
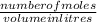
2.62 x 0.07 = number of moles
0.1834 moles of NaOH is there, to know the mass formula used is
mass = number of moles x atomic mass
= x 0.1834
= 7.33 grams of NaOH is the amount of conjugate base added.