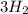 +
+
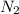 ⇔
⇔
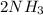
Decreasing the temperature of the reaction,the reaction shifts forward.
The explanation is given below.
Step-by-step explanation:
If the temperature of the reaction mixture is increased,then the equilibrium will shift to decrease the temperature.
If the temperature of the reaction mixture is decreased,then the equilibrium will shift to increase the temperature.
During the formation of the ammonia,it gives off heat.So it is an exothermic reaction.
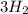 +
+
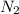 ⇔
⇔
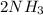
A decrease in the temperature favors the reaction that is exothermic (the forward reaction)because it produces energy.Therefore,if the temperature is decreased,the yield of the ammonia increases.
Therefore if the temperature is increased,the reaction shifts forward and the yield of the ammonia increases and it is an exothermic reaction.