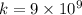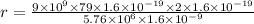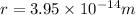Answer:
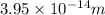
Step-by-step explanation:
We are given that
Charged on alpha particle=q=2e=
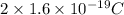
Where

Initial kinetic energy=K.E=5.76 MeV=
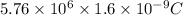

Z=79
Charge on protons=
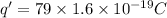
We have to find the closeness of alpha particle to the gold nucleus before being turned around.
Initial kinetic energy=Final potential energy
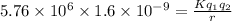
Where