Answer:
0.15cal/g°C
Step-by-step explanation:
Given parameters:
Mass of copper wire = 35g
T₁ = 29°C
T₂ = 76°C
Amount of heat absorbed = 243cal
Unknown:
Specific heat of copper = ?
Solution:
The specific heat of a substance is the amount of heat needed to raise the temperature of a unit mass of such substance by 1°C.
We can express this mathematically as;
C =
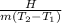
Where H is the heat supplied
C is the specific heat
m is the mass
T is the temperature(1 is the initial state and 2 is the final state)
Input the parameters and solve;
C =
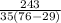 = 0.15cal/g°C
= 0.15cal/g°C