The answer for the following answer is mentioned below.
- Therefore the mass of calcium nitrate (
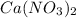
Step-by-step explanation:
To find the mass of calcium nitrate (
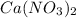 ) is :
) is :
No of calcium are 1
No of nitrogen are 2
No of oxygen are 6
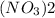 =
=
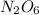
So therefore;
No of nitrogen are 2
No of oxygen are 6
Mass of (
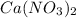 ) :
) :
molecular mass of calcium =40
molecular mass of nitrogen = 14
molecular mass of oxygen = 16
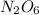 = (14×2) + (16×6)
= (14×2) + (16×6)
= 28 + 96
= 124
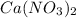 = 40 + 124
= 40 + 124
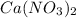 = 164 grams
= 164 grams
Therefore the mass of calcium nitrate (
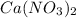 ) is 164 grams
) is 164 grams