The answers for the following questions is answered below.
- Therefore the volume of a gas is 22.4 L
- Therefore the number of moles of oxygen are 2.37 moles
Step-by-step explanation:
1. Given:
no of molecules (n) = 6.023 ×

volume of gas at STP conditions (V) =22.4 liters
To solve:
Volume of gas (v)
We know;
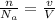
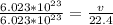
1 × 22.4 = v
v =22.4 L
Therefore the volume of a gas is 22.4 L
2. Given:
volume of oxygen gas (v) = 53 L
Volume of gas at STP conditions (V) =22.4 L
To solve:
no of moles of oxygen gas
We know;
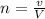
n =
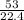
n =2.37 moles
Therefore the number of moles of oxygen are 2.37 moles