The given question is incomplete. The complete question is ;
Ammonia gas can be prepared by the following reaction:
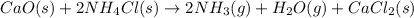
In an experiment, 27.6 g of ammonia gas,
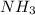 , is produced when it was predicted that 50.2 g of
, is produced when it was predicted that 50.2 g of
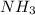 would form.
would form.
What is the theoretical yield of
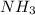 ? What is the actual yeild of
? What is the actual yeild of
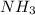 ? What is the percent yeild of
? What is the percent yeild of
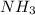 ?
?
Answer: a) 50.2 g
b) 27.6 g
c) 5.0 %
Step-by-step explanation:
a) Theoretical yield is defined as the amount of the product that is possible in the reaction.
Theoretical yield of
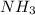 = 50.2 g
= 50.2 g
b) Experimental or actual yield is defined as the amount of the product that is actually formed in the reaction.
Experimental yield of
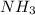 = 27.6 g
= 27.6 g
c) Percentage yield is defined as the ratio of experimental yileld to the theoretical yield in terms of percentage.
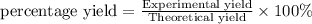
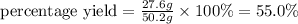
Hence percentage yield for the reaction is 55.0 %.