Answer:
* Limiting reagent: potassium.
*
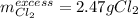
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
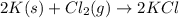
In such a way, the reacting moles of chlorine are:
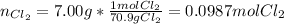
Now, the amount of chlorine gas that would react with 5.00 g of potassium result:
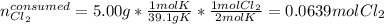
Thus, the chlorine will be in excess and the potassium will be the limiting reagent. Therefore, the mass of excess chlorine turns out:
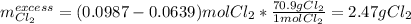
Best regards.