Answer:
The correct answer is option 4.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For exothermic reaction :ΔH = negative
- If the temperature is increased, so according to the Le-Chatlier's principle , the equilibrium will shift in the direction where decrease in temperature occurs.So, equilibrium will move in backward direction.
- If the temperature is decreased , so according to the Le-Chatlier's principle , the equilibrium will shift in the direction where increase in temperature occurs.So, equilibrium will move in forward direction.
For the given equation:
I)
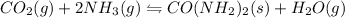 ,ΔH° = -90 kJ
,ΔH° = -90 kJ
On Increasing the temperature
This is an exothermic reaction, backward reaction will decrease the temperature. Hence, the equilibrium will shift in the left direction that is towards reactants.
II)
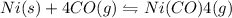 ,ΔH° = -161 kJ
,ΔH° = -161 kJ
On Increasing the temperature
This is an exothermic reaction, backward reaction will decrease the temperature. Hence, the equilibrium will shift in the left direction that is towards reactants.