Answer: The standard enthalpy of formation of liquid octane is -250.2 kJ/mol
Step-by-step explanation:
The given balanced chemical reaction is,
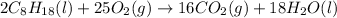
First we have to calculate the enthalpy of reaction
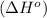 .
.
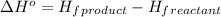
![\Delta H^o=[n_(O_2)* \Delta H_f^0_((O_2))+n_(H_2O)* \Delta H_f^0_((H_2O))]-[n_{C_8H_(18)}* \Delta H_f^0_{(C_8H_(18))+n_(O_2)* \Delta H_f^0_((O_2))]](https://img.qammunity.org/2021/formulas/chemistry/college/6y4ebcz6443f1so5p2r74msgpihmv40asp.png)
where,
We are given:
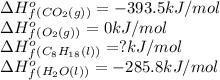
Putting values in above equation, we get:
![-1.0940* 10^4=[(16* -393.5)+(18* -285.8)]-[(25* 0)+(2* \Delat H_f{C_8H_(18)(l)}]](https://img.qammunity.org/2021/formulas/chemistry/college/ck1nyd4v7pwfz57ypgsu0us20w2xfselal.png)
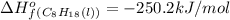
Thus the standard enthalpy of formation of liquid octane is -250.2 kJ/mol