Answer: The total volume of the gaseous products is 1044.29 L
Step-by-step explanation:
We are given:
Volume of butane = 116 L
At STP:
22.4 L of volume is occupied by 1 mole of a gas
So, 116 L of volume will be occupied by =
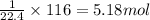 of butane
of butane
The chemical equation for the combustion of butane follows:
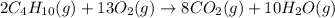
By Stoichiometry of the reaction:
2 moles of butane produces 8 moles of carbon dioxide
So, 5.18 moles of butane will produce =
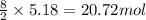 of carbon dioxide
of carbon dioxide
Volume of carbon dioxide at STP = (20.72 × 22.4) = 464.13 L
By Stoichiometry of the reaction:
2 moles of butane produces 10 moles of water vapor
So, 5.18 moles of butane will produce =
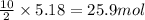 of water vapor
of water vapor
Volume of water vapor at STP = (25.9 × 22.4) = 580.16 L
Total volume of the gaseous products = [464.13 + 580.16] = 1044.29 L
Hence, the total volume of the gaseous products is 1044.29 L