Answer:
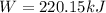
Step-by-step explanation:
Hello,
In this case, the work in terms of volume and constant pressure is:
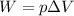
In such a way, since at the beginning there is no gas due to the fact that it is a gas-forming reaction, the work results:
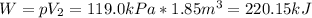
So no need of units conversion is present since kPa and cubic meters yields kJ.
Best regards.