Answer : The molarity of an acetic acid solution is, 0.0338 M
Explanation :
Formula used :

where,
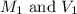 are the molarity and volume of acetic acid.
are the molarity and volume of acetic acid.
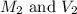 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.
We are given:
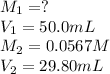
Putting values in above equation, we get:
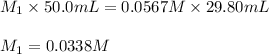
Hence, the molarity of an acetic acid solution is, 0.0338 M