Answer:
The minimum uncertainty in its position is 1.1587 nm
Step-by-step explanation:
Given;
average speed of electron, v = 5.00 × 10⁶ m/s
percentage of speed uncertainty = 1%
Δv = 0.01( 5.00 × 10⁶ m/s) = 5.00 × 10⁴ m/s
Applying Heisenberg's uncertainty principle, to determine the uncertainty in its position.
ΔxΔP ≥ h/4π
Δx(mΔv) ≥ h/4π
Δx = h/4πmΔv
where;
Δx is uncertainty in its position
h is Planck's constant
m is mass of electron
Δx ≥
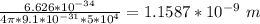
Δx ≥ 1.1587 nm
Therefore, the minimum uncertainty in its position is 1.1587 nm