Answer:
Moles of NO₂ = 0.158
Step-by-step explanation:
SO 2 ( g ) + NO 2 ( g ) ⇄ SO 3 ( g ) + NO ( g )
According to the law of mass equation
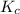 =
=
![([SO_(3) ][NO])/([SO_(2)][NO_(2) ])](https://img.qammunity.org/2021/formulas/chemistry/college/8fmc4kpxjnbu550g8b37jrrun7y6bv2qiq.png)
⇒ 3.10 =
![((1.00)(1.00))/((2.30) [NO_(2) ])](https://img.qammunity.org/2021/formulas/chemistry/college/obqb84w37ir1jlt35z3c5izc9y2z97ulc4.png) At equilibrium [SO₃] = [NO]
At equilibrium [SO₃] = [NO]
⇒ [NO₂] =
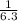
⇒ [NO₂] = 0.158
So. number of moles of NO₂ at equilibrium added = 0.158