Answer:
The value of the
 of the nitric acid is 1.2.
of the nitric acid is 1.2.
Step-by-step explanation:
The initial concentration of nitric acid = c = 7.50 M
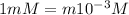
The dissociation constant of nitric acid =

Degree of dissociation of nitric acid =
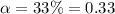
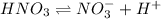
initially
c 0 0
At equilibrium
(c-cα) cα cα
The expression of dissociation constant :
![K_a=([NO_3^(-)][H^+])/([HNO_3])](https://img.qammunity.org/2021/formulas/chemistry/college/6vlvrlx8zc0rv6na0u8mlh2z3yiknrc96b.png)
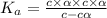
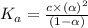
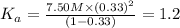
The value of the
 of the nitric acid is 1.2.
of the nitric acid is 1.2.