Answer: The equilibrium concentration of chloride ions in the solution is 4.532 M
Step-by-step explanation:
We are given:
Initial molarity of
![[CoCl_2.6H_2O]](https://img.qammunity.org/2021/formulas/chemistry/college/2hkr3gem1oy3lkdpefycubvg17o6etty67.png) = 0.054 M
= 0.054 M
Initial molarity of HCl = 4.60 M
Equilibrium molarity of
![[CoCl_4]^(2-)](https://img.qammunity.org/2021/formulas/chemistry/college/l4znrhjni1li466d55i4rv0hbqqdagems2.png) = 0.034 M
= 0.034 M
The chemical equation for the reaction of
![[CoCl_2.6H_2O]](https://img.qammunity.org/2021/formulas/chemistry/college/2hkr3gem1oy3lkdpefycubvg17o6etty67.png) and HCl follows:
and HCl follows:
![[CoCl_2.6H_2O]+2HCl\rightarrow H_2[CoCl_4]+6H_2O](https://img.qammunity.org/2021/formulas/chemistry/college/4zxnrdzmr2f1t6d7t1hzpbc2wkh4ty42fz.png)
Or,
![[CoCl_2.6H_2O]+2Cl^-\rightarrow [CoCl_4]^(2-)+6H_2O](https://img.qammunity.org/2021/formulas/chemistry/college/8pbmfsg3jbivmjyax9o5fyzffcnu6nz7gf.png)
Initial: 0.054 4.60
At eqllm: 0.054-x 4.60-2x x 6x
Evaluating the value of 'x':
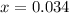
So, equilibrium concentration of
 = (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M
= (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M
Hence, the equilibrium concentration of chloride ions in the solution is 4.532 M