Answer:
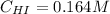
Step-by-step explanation:
Hello,
In this case, based on the given units of the rate constant, it is a first-order reaction with respect to HI, therefore, the rate law is:
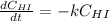
Whose integration turns out:
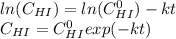
Therefore, the concentration of HI after 800 second finally result:
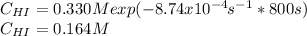
Best regards.