Step-by-step explanation:
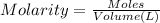
1) Molarity of the chloride solution = 2.5 M
Volume of the chloride solution = 100 mL = 0.100 L ( 1mL = 0.001 L)
Moles of chloride ions = n
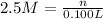
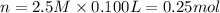
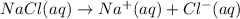
1 mole of chloride ions are obtained from 1 mole NaCl,then 0.25 mol of chloride ions will be obtained from :
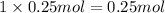 of NaCl
of NaCl
Mass of 0.25 moles of NaCl :
0.25 mol × 58.5 g/mol = 14.62 g
In a dry 100 mL volumetruc flask add weighed 14.62 grams of NaCl and small amount of water to dissolve it completely. After this add the water upto mark of 100 mL to form chloride solution of 0.25 M.
2) Molarity of the chloride solution = 0.5 M
Volume of the chloride solution = 100 mL = 0.100 L ( 1mL = 0.001 L)
Moles of chloride ions = n
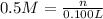
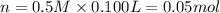
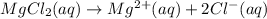
2 mole of chloride ions are obtained from 1 mole
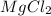 ,then 0.05 mol of chloride ions will be obtained from :
,then 0.05 mol of chloride ions will be obtained from :
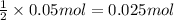 of
of
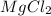
Mass of 0.025 moles of
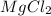 :
:
0.025 mol × 95 g/mol = 2.375 g
In a dry 100 mL volumetruc flask add weighed 2.375 grams of
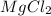 and small amount of water to dissolve it completely. After this add the water upto mark of 100 mL to form chloride solution of 0.5 M.
and small amount of water to dissolve it completely. After this add the water upto mark of 100 mL to form chloride solution of 0.5 M.