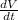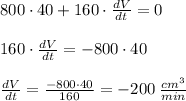Answer:
The volume decreasing at the rate of
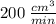 .
.
Explanation:
We need to find at what rate is the volume decreasing
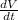 , when
, when
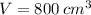 ,
,
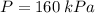 , and
, and
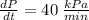 .
.
We know that when a sample of gas is compressed at a constant temperature, the pressure P and volume V satisfy the equation
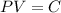 , where C is a constant.
, where C is a constant.
So, we can differentiate this equation with respect the time. For this we use the Chain Rule
![\frac{d}{{dx}}\left[ {f\left( u \right)} \right] = \frac{d}{{du}}\left[ {f\left( u \right)} \right]\frac{{du}}{{dx}}](https://img.qammunity.org/2021/formulas/mathematics/college/t5d0f00wb1ed7zvznyyovoqj14ve7qxpoc.png)
and the Product rule
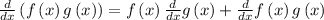
to differentiate both sides with respect to time.
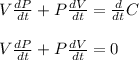
Next, we substitute the given values
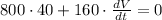
and we solve for