As the pressure of the container in the furnace (3.64 atm) is less than the withstanding pressure of the container (45 atm) , it will not burst.
Step-by-step explanation:
As per the Guy-Lusac's law, if the volume occupied by a gas molecules are kept constant, then there will be increase in pressure with increase in temperature. This means the pressure exerted by gas molecules will be directly proportional to temperature, if the volume is kept constant.
So in the present case, the initial pressure of the container is 1.5 atm and the temperature in Kelvin is (25 +273 ) 298 K. When it is thrown in furnace, the temperature of the container will get increased to the temperature of furnace i.e., 450+273 = 723 K. Then the new pressure can be obtained as follows.
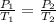
Since, P₁ = 1.5 atm, T₁ = 298 K, T₂ = 723 K, we need to find P₂
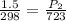
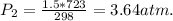
So, the pressure of the container will get increased to 3.64 atm when it is thrown into a furnace. Thus, the container will not burst in the furnace as the container can withstand a pressure of upto 45 atm. So as the pressure of the container in the furnace is less than the withstanding pressure of the container, it will not burst.