Answer:
1) Increasing the concentration of
 shift the equilibrium to the right side.
shift the equilibrium to the right side.
2) Increasing the concentration of
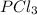 shift the equilibrium to the left.
shift the equilibrium to the left.
3) increasing the pressure by adding argon gas to the reaction mixture, while maintaining a constant volume will have no effect.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
On increasing concentration of reactants :
When concentration of reactants are increased the equilibrium shifts in forward direction(right) to reestablish itself and concentration of products increases with it.
On increasing concentration of products :
When concentration of products are increased the equilibrium shifts in backward direction (left) to reestablish itself and concentration of reactants increases with it.
On adding inert gas at constant volume.
No change will occur because adding inert gas will increase the total pressure of the system not the partial pressure of the components of the equilibrium.
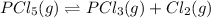
1) Increasing the concentration of
 shift the equilibrium to the right side.
shift the equilibrium to the right side.
2) Increasing the concentration of
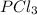 shift the equilibrium to the left.
shift the equilibrium to the left.
3) increasing the pressure by adding argon gas to the reaction mixture, while maintaining a constant volume will have no effect.