15.76 grams of AgCl will be produced from 7.00 g of NaCl and 95.0 g of AgNO3.
Step-by-step explanation:
Balanced equation for the reaction:
NaCl + AgNO3 → AgCl + NaNO3
given:
mass of NaCl = 7 gram
mass of AgNO3 = 90 GRAM
atomic mass of NaCl = 58.44 grams/mole
atomic mass of AgNO3 = 169.87 grams/mole
number of moles =
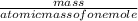 1 equation
1 equation
putting the values in equation 1
number of moles of NaCl =
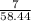
= 0.11 moles
number of moles of AgNO3 =
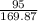
= 0.55
the limiting reagent is NaCl as it is a reactant that produces small quantity of AgCl
1 Mole of NaCl reacted to form 1 mole of AgCl
0.11 mole of NaCl will produce x moles of AgCl
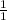 =
=
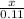
0.11 moles of AgCl is produced
atomic mass of AgCl = 143.32
mass = 0.11 x 143.32
= 15.76 grams of AgCl is produced.