Answer:
16g
Step-by-step explanation:
Given parameters:
Mass of H₂ = 2.0g
Mass of O₂ = 16g
Unknown:
Mass of oxygen that will react to form H₂O₂ = ?
Solution:
Let us establish the reaction equation first;
H₂ + O₂ → H₂O₂
This equation is balanced chemical equation;
Now to solve this problem, find the limiting reactant. The limiting reactant is the reactant in short supply.
To find this reactant, convert the given masses to number of moles.
Number of moles =

Molar mass of H₂ = 2(1) = 2g/mol
Molar mass of O₂ = 2(16) = 32g/mol
Number of moles of H₂ =
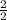 = 1mole
= 1mole
Number of moles of O₂ =
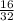 = 0.5mole
= 0.5mole
From the balanced reaction equation;
1 mole of H₂ reacted with 1 mole of O₂
Therefore 1 mole of H₂ will require 1 mole of O₂
But the given number of O₂ is 0.5moles. This is the limiting reactant that will determine the extent of the reaction.
The mass of 0.5moles of oxygen gas is 16g