Step-by-step explanation:
Chemical equation for the given reaction is as follows.
![[Co(H_(2)O)_(6)]^(2) + 4Cl^(-) \rightleftharpoons [CoCl_(4)]^(2-) + 6H_(2)O](https://img.qammunity.org/2021/formulas/chemistry/college/mwnk3w2tj768h49d0f97ajq6i54eenk4mi.png)
First, we will calculate the moles of
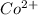 as follows.
as follows.
![(0.652 * 1 mole Co^(2+))/(1 mole [CoCl(H_(2)O)_(6)]^(2+))](https://img.qammunity.org/2021/formulas/chemistry/college/9yhyv5pwo7ne7sa4ovoeqtx06w884iqk0h.png)
= 0.652 mol
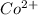
Therefore,
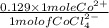
= 0.129 moles of
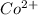
Now, moles per liter of
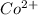 which will not react will be calculated as follows.
which will not react will be calculated as follows.
0.652 - 0.129
= 0.523
Thus, we can conclude that there are 0.523 moles of
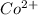 present per liter.
present per liter.